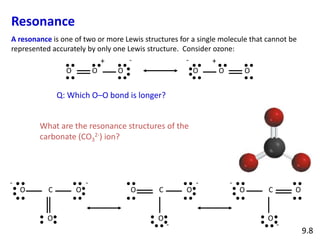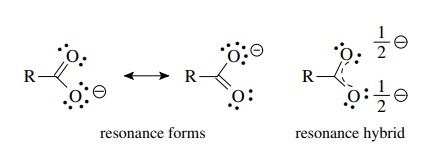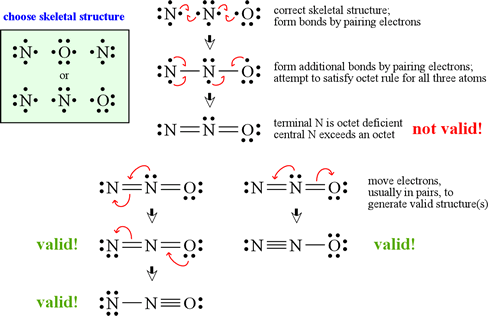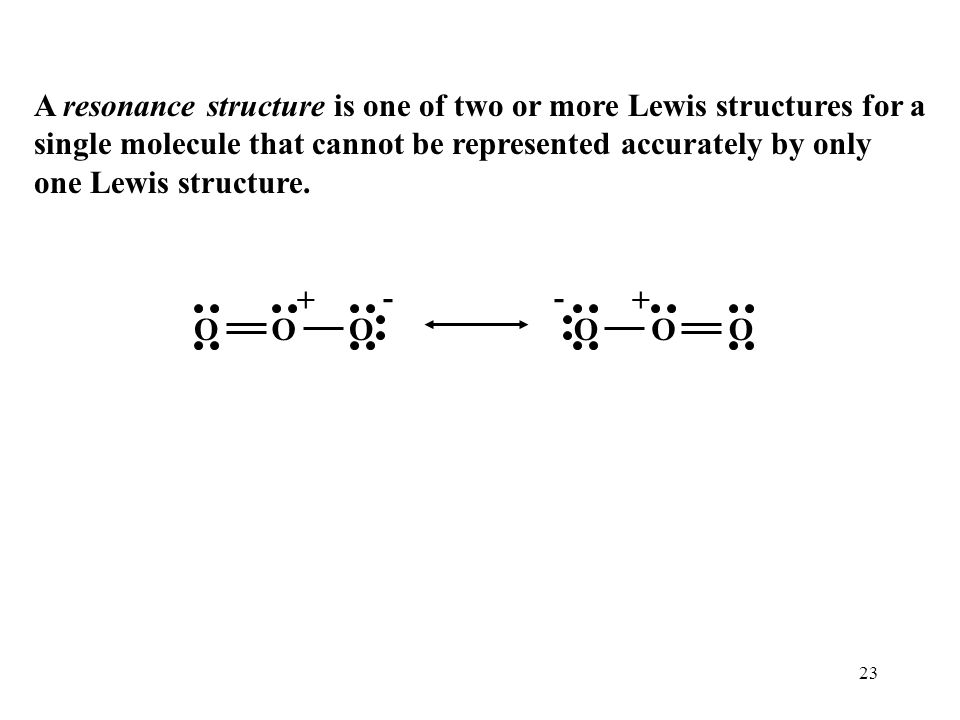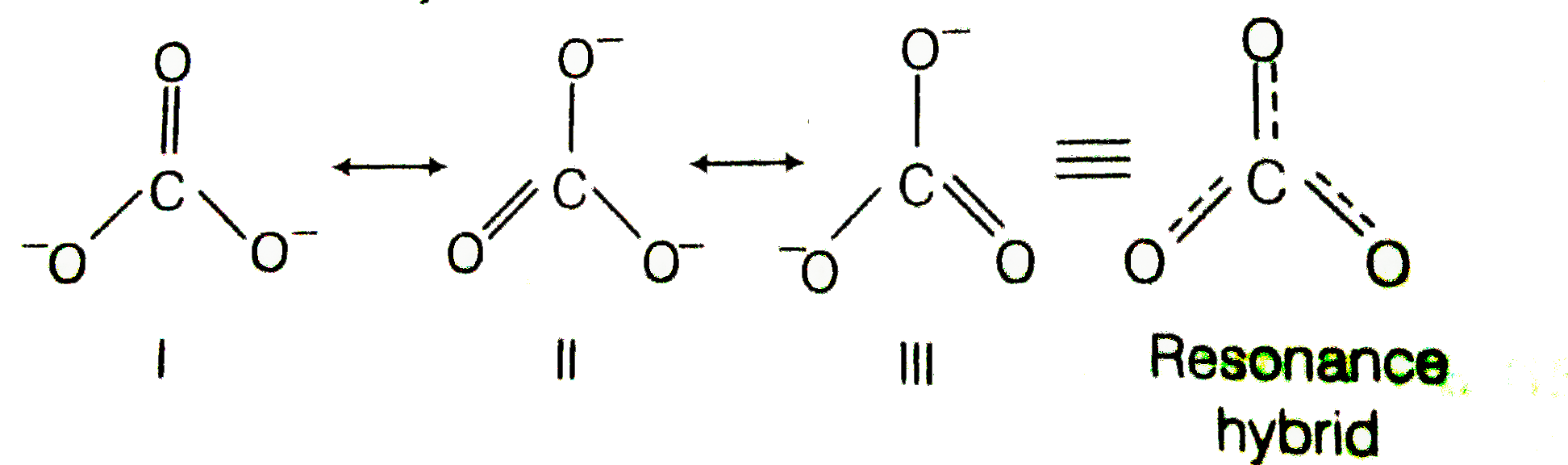
Explain why CO_(3)^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?

Simplified S 0 singlet PES at the G3SX//M06-2X/aug-cc-pVTZ level of... | Download Scientific Diagram

Explain why CO_(3^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?

Explain why CO_(3)^(2-) ion cannot be represented by a single Lewis structure. How can it be best represented?
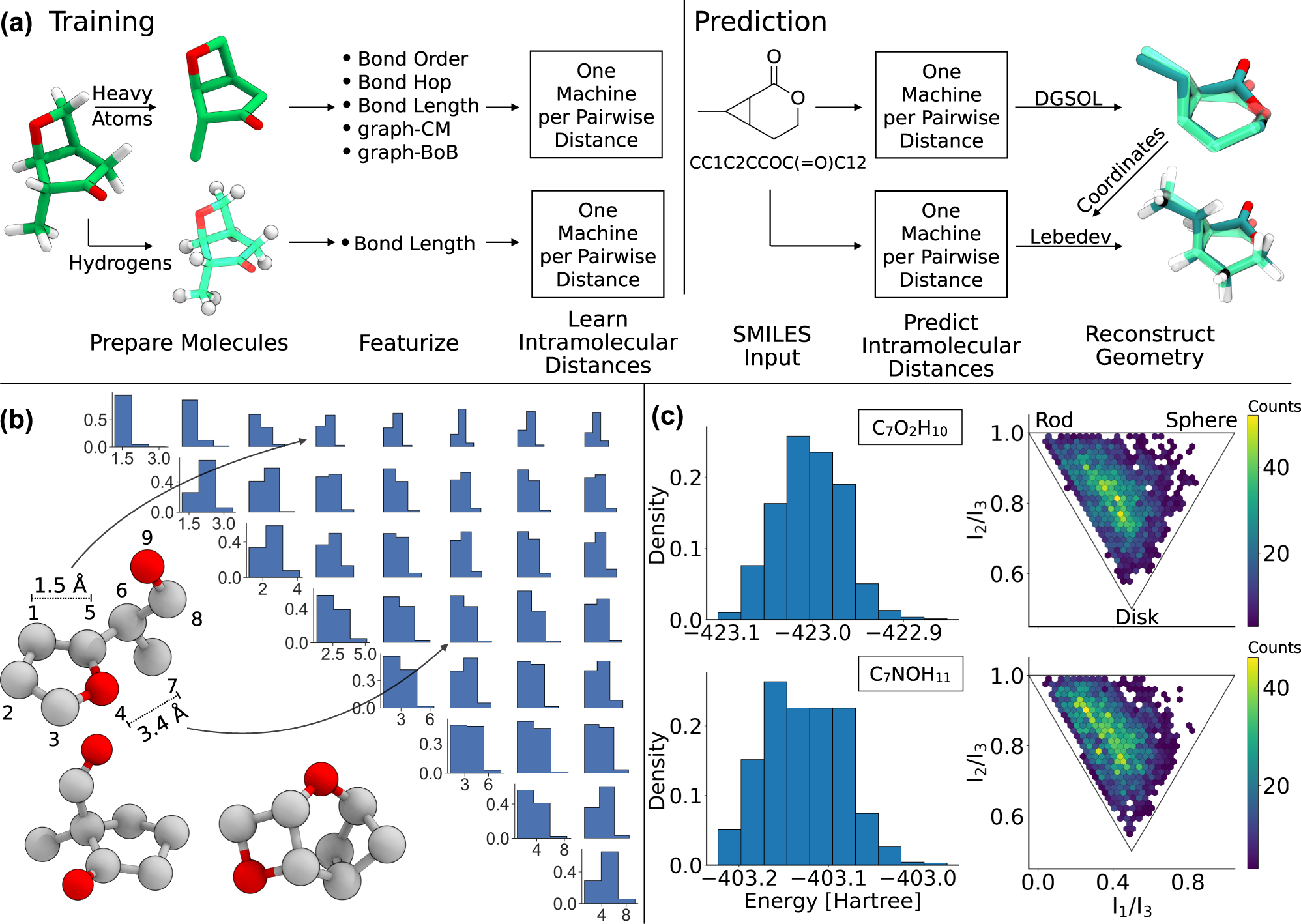
Machine learning based energy-free structure predictions of molecules, transition states, and solids | Nature Communications

Basic Concepts of Chemical Bonding Chapter 8. Three Types of Chemical Bonds Ionic bond Ionic bond –Transfer of electrons –Between metal and nonmetal ions. - ppt download

All questions go with Model 4 , please label thank you in the Ltwis suettre for PCls and XeFe Model 4: Which Lewis Structure is Better? The two structures below could represent

Explain why CO_{3}^{2-} ion cannot be represented by a single Lewis structure. How can it be best represented?
Simplified T 1 triplet PES at the G3SX//M06-2X/aug-cc-pVTZ level of... | Download Scientific Diagram

/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)